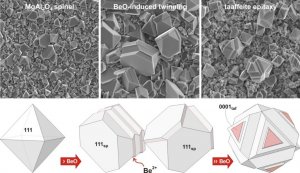
(111) twins in MgAl2O4 spinel
For a long time it was believed that twinning in spinel is a result of accidental attachment of crystallites in the initial stage of crystal growth (during nucleation). In fact, (111) twins in spinel form as a result of beryllium incorporation into the twin boundary plane. The local structure of the twin boundary is closely related to the structure of ternary Mg-Al-Be oxides also known as taaffeites. This type of twinning is chemically-induced or tropochemical twinning.
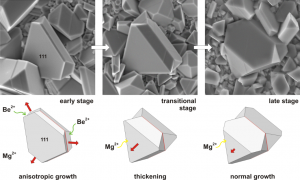
Chemically induced twins have important influence on crystal growth – after nucleation of the twin boundary, growth of the composite crystal proceeds exaggeratedly first in the direction of the twin boundary, whereas thickening occurs in the next growth stage:
N. Daneu, A. Rečnik, T. Yamazaki, T. Dolenec: Structure and chemistry of (111) twin boundaries in MgAl2O4 spinel crystals from Mogok. Physics Chemistry of Minerals 34 (2007) 233‐247. doi: 10.1007/s00269-007-0142-1
S. Drev, A. Rečnik, N. Daneu: Twinning and epitaxial growth of taaffeite-type modulated structures in BeO-doped MgAl2O4. CrystEngComm 15 (2013) 2640-2647. doi: 10.1039/C3CE26997C.
Contact:
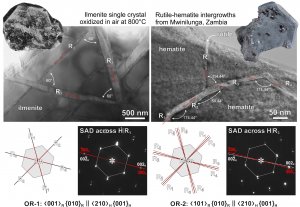
Oxidation of ilmenite, FeTiO3
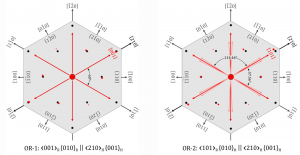
Oxidation of ilmenite to rutile and hematite is a typical example of topotaxial phase transformation – a solid-state reaction, where the initial single crystal is transformed into one or more products along definite crystallographic directions, leading to the formation of oriented 3D intergrowths. The result of ilmenite oxidation are rutile-hematite intergrowths. Two orientations of rutile exsolutions are possible, with the c-axes or [101] directions of rutile parallel to the a-axes of ilmenite (OR-1 and OR-2):
The direction of rutile exsolutions from ilmenite depends on the kinetics; fast oxidaiton leads to the development of OR-1, whereas OR-2 develops during slow transformaiton (low oxygen fugacity). The OR-1 developed during heating of ilmenite at 700-800 °C in air, while OR-2 was observed on natural rutile-hematite intergrowths from Mwinilunga in Zambia.
A. Rečnik, N. Stanković, N. Daneu (2015) Topotaxial reactions during the genesis of oriented rutile/hematite intergrowths from Mwinilunga (Zambia). Contrib. Mineral. Petrol. 169:19., doi: 10.1007/s00410-015-1107-x
Stanković N, Rečnik A, Daneu N (2016) Topotaxial reactions during oxidation of ilmenite single crystal. J Mater Sci 51: 958-968. doi: 10.1007/s10853-015-9425-y
Contact:
Twinning in rutile TiO2
Natural rutile crystals commonly occur in the form of oriented intergrowths or sagenites, where the angles between the rutile domains frequently correspond to crystallographic (coherent) angles of 114.4° or 57.2° as for the (101) or (301) twins. Well-developed rutile twins usually form under hydrothermal conditions as a consequence of epitaxial growth on a suitable substrate mineral like hematite or ilmenite.
(301) rutile twins: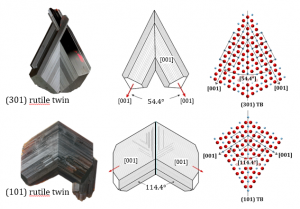
(101) rutile twins: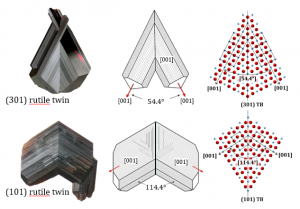
The main characteristic of epitaxial/topotaxial twins is that the twin interface contains a thicker layer of some secondary phase, precipitates or is devoid of any dopant (clean contact). (301) twin boundaries in rutiles from Diamantina contain a coherent layer of ilmenite, while the (101) twin contacts oriented precipitates of corundum. Evidences for transformation from oxyhydroxide precursor phase were found in both cases.
Daneu N, Rečnik A, Mader W (2014) Atomic structure and formation mechanism of (101) rutile twins from Diamantina (Brazil). Am Min 99:612–624. doi: 10.2138/am.2014.4672
Daneu N, Schmid H, Rečnik A, Mader W (2007) Atomic structure and formation mechanism of (301) rutile twins from Diamantina (Brazil). Am Min 92:1789–1799. doi: 10.2138/am.2007.2634
Contact:
Analytical electron microscopy of extraterrestrial materials
Meteorites are rare and precious findings because they reveal important information about the early formation of the solar nebula some 4.6 billion years ago, till very recent times, when they fell to the Earth’s surface. Systematic studies by means of advanced techniques of Analytical Electron Microscopy is being conducted on two stony meteorites, found in Slovenia; meteorite Jesenice and Jezersko.
Second research topic is related to the understanding of the early-stage crystallization pathways of the interstellar dust, which is achieved by specially designed micro gravity experiments where dust particles are homogeneously nucleated from supersaturated vapors, thus mimicking astronomically relevant conditions in the laboratory. Combined in-situ nucleation and high-resolution structure-chemistry studies of synthesized dust particles are providing detailed insights into the complexities of interstellar dust formation.